VIDEO: The Role of Physicists in Diagnostic Imaging
Philips launches Ingenia Ambition X 1.5T MR
Philips, a global leader in health technology, launched the Ingenia Ambition X 1.5T MR. This innovation is the latest advance in the Ingenia MRI portfolio, which comprises fully-digital MRI systems, healthcare informatics and a range of maintenance and life cycle services for integrated solutions that empower a faster, smarter, and simpler path to enabling a confident diagnosis. The first commercial installation of the Ingenia Ambition X was recently completed at Spital Uster Hospital, a major provider of extended primary healthcare in the canton of Zurich, Switzerland. The Ingenia Ambition X is CE marked and has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
In radiology, meeting the need for high productivity and an improved patient experience while ensuring excellence in imaging can be daunting. The perception is often that MR represents a trade-off between productivity and image quality. The Ingenia Ambition X provides leading-edge MR imaging capabilities while helping to increase overall productivity, combining its revolutionary BlueSeal magnet with innovations that can help reduce downtime, enable single operator workflow and speed up exam times by up to 50%.
"MRI provides exceptional diagnostic and therapy guidance capabilities, but it also places substantial operational demands on the hospital or imaging center due to requirements for installation, footprint and service," said Arjen Radder, Global Business Leader for MR at Philips. "BlueSeal is breakthrough MRI technology and we're proud to be first to market. The fully-sealed magnet dramatically reduces the amount of liquid helium needed to cool the magnet to less than half a percent of the current norm. This results in significant operational benefits for our customers, including a smaller, lighter and more flexible installation footprint and a more efficient return to normal operations if an interruption in service should ever occur."
Incorporating Philips' breakthrough BlueSeal fully-sealed magnet, the Ingenia Ambition X is the world's first MR system to enable helium-free operations [3], reducing the chance of potentially lengthy and costly disruptions, and virtually eliminating dependency on a commodity with an unpredictable supply. The fully-sealed system does not require a vent pipe and is around 900 kg lighter than its predecessor, significantly reducing the siting challenges presented by conventional magnets and lowering construction costs.
"We are very proud to be the first hospital in the world to offer this new cutting edge, resource-friendly MRI technology to our patients," said Andreas Steinauer, M.D., Chief Radiologist at Spital Uster Hospital, Switzerland. "With the new Philips Ingenia Ambition X our patients can have the best of two worlds: leading MRI technology with a smaller footprint. This new leaner platform will allow more sites to deliver advanced MRI technology to their patients, helping to improve patient care."
Wealth of innovations delivers a step-change in MR productivity
The Ingenia Ambition X includes a range of innovative features that combine to deliver a step-change in productivity. With Philips' EasySwitch solution, the BlueSeal's magnetic field can be easily turned off if an item becomes stuck in the bore. Once the problem is resolved, an in-house or Philips technician can initiate an automated ramp-up to bring the magnet back to field, minimizing operational downtime. A conventional MR can require two staff to manage daily operations. The Ingenia Ambition X combines guided patient setup and Adaptive Intelligence-driven SmartExam analytics for automatic planning, scanning and processing. This frees up time to enable a single operator to manage the full scan from the patient's side with just a single touch of a button.
Philips Compressed SENSE is an advanced acceleration application that reduces exam times by up to 50%. In addition, Philips VitalEye is a unique approach to detecting patient physiology and breathing movement. VitalEye technology and algorithms intelligently extract signs of breathing – allowing routine exam set-up time to occur in less than a minute, even for less experienced operators. Together, these innovations help to standardize and speed up workflow, allowing clinicians to focus on the patient.
Breaking down diagnostic boundaries by delivering speed, comfort and confidence
The Ingenia Ambition X is part of the all-new Ingenia digital MR portfolio. Find out more about how Philips' MR innovations are improving speed, comfort and confidence here. For more information on how Philips is helping to break down silos and provide solutions that improve the patient and staff experience, drive better productivity and efficiency, enable increased diagnostic confidence and better practice management, visit here.
Dose-lowering Practices for CCTA

An example of the newest generation of smart cardiac CT software that automatically identifies the anatomy, autotraces the centerlines on the entire coronary tree and labels each vessel segment. This greatly speeds CT workflows, saving time for techs, radiologists and cardiologists.
Here is a checklist of dose-sparing practices for cardiac computed tomography (CT) imaging used in the cath lab. This list was included in a 2018 consensus document to guide the optimal use of ionizing radiation in cardiovascular imaging.1
The consensus document was issued in May 2018 jointly by the American College of Cardiology (ACC), Heart Rhythm Society (HRS), North American Society for Cardiovascular Imaging (NASCI), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the Society of Cardiovascular Computed Tomography (SCCT).
It includes input from experts from a total of nine cardiology societies, and includes best practices for safety and effectiveness when using computed tomography (CT), nuclear imaging and angiographic/fluoroscopic imaging.
One section includes the following checklist to help lower dose in cardiovascular CT angiography (CCTA):
Case Selection:
• Consider patient age, co-morbidities, natural life expectancy; and
• Consider appropriateness and utility of nonradiation-based imaging techniques.
Equipment Calibration:
• Use acquisition detector doses as low as compatible
with diagnostic image quality.
Procedure Planning:
• Select lowest-dose acquisition protocol compatible with study goals;
• Use ECG-gated variable tube output if retrospective gating is used;
• Use the lowest X-ray tube voltage compatible with adequate diagnostic-quality image acquisition;
• Use the lowest X-ray tube current compatible with diagnostic-quality image acquisition; and
• Use the largest scan pitch compatible with adequate diagnostic-quality image acquisition.
Study Conduct:
• Minimize patient heart rate; and
• Confine scanned body area to the area relevant to the study’s diagnostic purpose.
Variables That Affect Patient Dose for X-ray CT
The radiation dose to a patient is determined by a combination of the patient’s physical characteristics and scanner protocol selection. Patient exposure will necessarily increase with patient size and body mass index. Depending on the specific acquisition parameters, the increased exposure need not increase dose to radiosensitive tissues. Patient size is not a variable that determines exam appropriateness as long as the patient’s size does not preclude obtaining diagnostic-quality images.
X-ray CT systems can either use a constant X-ray tube output or, in some acquisition protocols, use ECG-gated variable output. The operator selects the acquisition protocol based on patient characteristics and the study purpose, with the intent to deliver a sufficient exposure to permit an acceptable degree of noise in the reconstructed images.
The X-ray CT system operator is responsible to select the scanning protocol that optimizes the examination’s diagnostic yield while minimizing dose. The following are essential considerations in this process:
1.Scan length. Scan length, defined as the distance imaged along the cranio-caudal axis, should be kept to a minimum to encompass only the anatomy of interest and not expose structures that are not relevant to the examination’s purpose. Care needs to be taken to ensure that the diaphragm position seen on the topogram is the same as during the scanning. This requires similar breath-hold instructions.
2. X-ray beam intensity. X-ray beam intensity is determined by both the X-ray tube potential (in units of kV) and the X-ray tube current (in units of mA). Modern
CT scanners modulate the tube current dynamically throughout the CT acquisition to minimize radiation exposure.
Tube potential: Studies of radiation dose reduction have demonstrated that the most important single factor in controlling radiation dose is adjustment of X-ray tube voltage. Increasing tube voltage increases the X-ray beam’s mean photon energy level, and increases radiation dose roughly proportionally to the square of the voltage. Thus, at a constant tube current, a decrease of tube voltage from 120 to 100 kV reduces the radiation dose by almost 40 percent. In most scanners, the X-ray tube voltage may be adjusted between
70 to 140 kilovolts (kV). The voltage is selected by the operator based on subject weight or body mass index. A commonly used adjustment scale that provides diagnostic quality in most scanners is: 120 kV for patients with body mass index ≥30 kg/m2, 100 kV for body mass index 21 to 29 kg/m2, and 80 kV for body mass index <21 kg/m2. Image noise decreases as potential increases, so that in extreme cases (body mass index ≥40 kg/m2) the maximum tube potential of 150 kV may be necessary
to produce diagnostic-quality images.
Tube current: The X-ray tube current (in mA) is defined as the number of electrons accelerated across the tube per unit of time and is proportional to the number of X-ray photons produced per unit time. The radiation dose is linearly proportional to the tube current. Image noise is inversely proportional to the square root of the tube current. Thus, decreasing tube current at a given tube potential decreases the radiation dose at the expense of increased image noise. The tube current may be modified based on patient size assessed by visual inspection, measurement of body weight or body mass index, thoracic circumference or diameter, or noise measurement from a cross-sectional prescan or topogram. Most modern scanners offer tube current modulation based on the thickness of the body estimated from the topogram. Modulation may be applied longitudinally as well as circumferentially. This approach can reduce radiation exposure of thoracic CT examinations by 20 percent without increasing image noise.
3. Rotation time. The time required for the gantry to perform one rotation is a selectable parameter. Exposure increases linearly with rotation time.
4. X-ray beam filtration. Filters placed beneath the X-ray tube are used to selectively attenuate low-energy X-rays that do not significantly contribute to the image but do contribute to radiation dose. The net effect is to increase the mean energy of the X-rays while not altering the maximum energy.
5. Scan acquisition mode. This is a major determinant of radiation dose. Different acquisition modes can deliver substantially different doses while producing similar images. There are three principal CT scan modes: axial or “conventional” scanning, helical scanning, and fixed-table or single-station scanning.
6. Cardiac motion compensation. Compensation for cardiac motion is rarely applied outside of direct cardiac and aortic root imaging. Thus, the majority of cardiovascular medical imaging does not employ ECG gating or triggering. In contrast, when imaging the heart or aortic root, cardiac motion compensation is critical to avoiding motion-related artifacts that substantially degrade image quality. Depending upon the scan mode, one of two cardiac compensation methods is used:
Prospective ECG triggering: Prior to the scan, the operator “prospectively” selects an imaging window within the cardiac cycle, which may be defined as a percentage from one R-wave to the next, or an absolute time delay after each R-wave. Scans are then triggered to coincide with the selected scan window. Prospective triggering may be applied to each of the three scan modes. In the case of axial scanning, ECG triggering is used to trigger the acquisition at each table position.
Retrospective gating: Applicable to both helical and fixed table scan modes. With helical scanning, acquisition is performed using a low pitch of approximately 0.2. The slow acquisition images the entire cardiac anatomy across the entirety of a cardiac cycle, providing a 4-D dataset that allows each spatial location within the heart to be reconstructed at any time-point across the cardiac period. Data is continuously acquired along with the ECG signal while covering the anatomy of interest. The data is subsequently rebinned at each slice location for image reconstruction, according to the time of the cardiac cycle from the ECG signal.
ECG-triggered tube current modulation: As discussed in the preceding section, “tube current,” ECG-triggered tube current modulation is used to reduce radiation dose during systole when there is the greatest cardiac motion and can reduce the radiation exposure significantly. In this circumstance, tube current is at nominal value only during the portion of the cardiac cycle likely to be used for reconstruction (typically end diastole). During the remainder of the cardiac cycle, the tube current is reduced to minimize radiation output. Recent refinements of this technique have allowed reduction of the length of time (“window”) during which tube current is nominal and reduction of tube current during the undesired portions of the cardiac cycle by 20 percent and to as little as 3 to 5 percent of the nominal value. A potential disadvantage of this technique is that images reconstructed from projection data acquired with low tube current may be too noisy to be diagnostic for coronary anatomy. Retrospectively ECG-triggered tube current modulation works best in patients with stable sinus rhythm and low heart rates (specific thresholds depend on scanner characteristics).
7. Image reconstruction. Filtered back-projection has historically been used to reconstruct CT images from projection data. The advent of greater computing power has made an alternative statistical method — iterative reconstruction — practical for CT. This method predicts projection data based on an initial assumption about the attenuation in each voxel, and compares that data to measured projection data. The voxel attenuation values are modified iteratively until an acceptable level of error between the predicted and measured data is obtained. The resulting reconstructed images have lower noise values compared with those obtained with filtered back projection. This permits reducing tube voltage and/or current to obtain images with comparable noise and lower radiation dose. One important characteristic of iterative reconstruction is that excessively low-dose images do not appear grainy, as is the case with filtered back projection. Instead, structures become blurred and can develop a blotchy appearance, undermining their diagnostic effectiveness.
8. Image post-processing filters. These may also be applied to acquired images to reduce image noise while preserving image contrast and edges. The feasibility of using these filters for radiation dose reduction has been recently demonstrated.
Reference:
1. John W. Hirshfeld Jr., Victor A. Ferrari, Frank M. Bengel, et al. 2018 ACC/HRS/NASCI/SCAI/SCCT Expert Consensus Document on Optimal Use of Ionizing Radiation in Cardiovascular Imaging: Best Practices for Safety and Effectiveness: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. Journal of the American College of Cardiology. May 2018. DOI: 10.1016/jacc.2018.02.016.
How Digital PET/CT Can Improve Clinical Care
Bone Marrow Edema in Traumatic Vertebral Compression Fractures
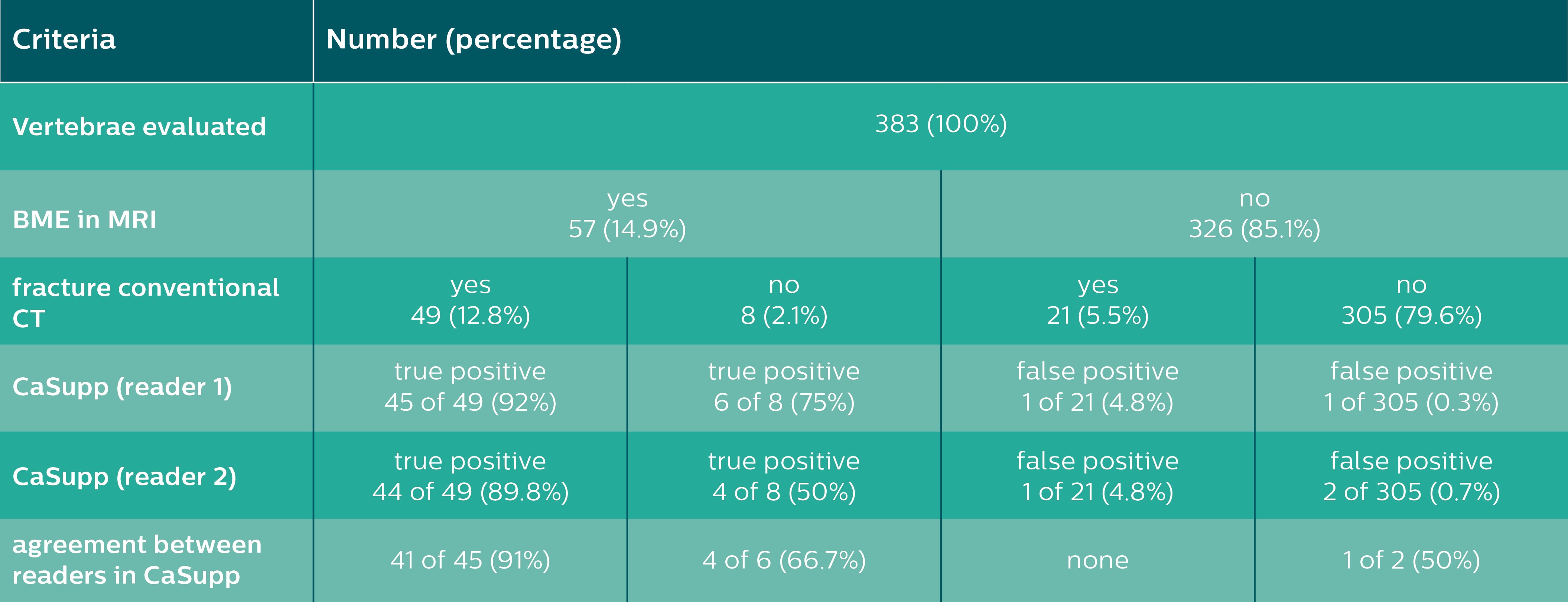
Results of the vertebrae-based analysis (383 vertebrae in 34 patients) for detection of BME.
The following is a summary of a study published in the European Journal of Radiology, 105 (2018) 216–220, by Victor Neuhaus, M.D., and colleagues from the University Hospital of Cologne, Cologne, Germany
The purpose of the study was to evaluate the use of calcium suppression images (CaSupp) provided by Philips IQon Elite Spectral CT to enhance the diagnostic accuracy of detecting bone marrow edema (BME) in vertebral fractures (VF).
Overview
Vertebral fractures are common and require individual treatment, as they are associated with considerable morbidity, mortality and future fractures. Therefore, a precise diagnostic workup of vertebral fractures is crucial for adequate clinical management.
In clinical routine, X-ray and CT of the spine are performed as the initial assessment of VF. However, the dense trabecular bone can obscure the visualization of post-traumatic bone marrow changes, making MRI the modality of choice to differentiate acute and chronic VF based on the presence or absence of BME. But one of the limitations of using an MRI for this assessment is an MRI’s limitation in helping the clinician adequately visualize trabecular and cortical bone structures. This means that assessing both high contrast (trabecular bone) and low contrast tissues (bone marrow) in one examination can be a challenge.
To address this issue, IQon Elite Spectral CT introduced a CaSupp image to its list of spectral results available for every patient scanned on an IQon Elite. With these images, voxels containing calcium are suppressed and replaced by virtual HU values, as similar as possible to the expected HU, without the calcium contribution to the attenuation. The extent of calcium suppression can be modified using different calcium suppression indices. Bone marrow changes, normally obscured by the dense trabecular bone, may be visualized and therefore allow for the detection of traumatic BME.
Methods
This study involved a retrospective analysis of 34 patients, scanned on the IQon Elite Spectral CT, a dual-layer spectral detector CT (SDCT). All of these patients were diagnosed with at least one vertebral fracture and had undergone a complementary MRI. The MRI images were evaluated in order to detect vertebrae with traumatic BME.
Two readers, who were blinded to the results of MRI, evaluated CaSupp for vertebral BME and determined the optimal calcium suppression indices for visualization of BME. In addition, bone mineral density was assessed based on ROI measurements. Moreover, one reader measured the attenuation and standard deviation of BME in fractured and normal vertebrae previously determined in MRI using ROIs in the CaSupp images. The measurements were conducted at different CaSupp indices, ranging from 30 to 100 with an increment of 10 in order to calculate contrast-to-noise ratios.
For the results of the detection of BME, specificity, sensitivity, positive and negative predictive value, and accuracy were calculated. Statistical tests were performed for interrater agreement and contrast-to-noise (CNR) analysis.
Results
A total of 383 vertebrae were assessed in 34 patients. MRI revealed BME in 57 out of 383 vertebrae (14.9 percent). Of the 57 vertebrae showing BME in MRI, both readers detected 51 and 48 true positive vertebrae with BME in CaSupp, respectively. In contrast to that, two and three false positive findings of BME were reported by Reader one and Reader two (see Table 1).
Overall, CaSupp yielded an average sensitivity of 87 percent and specificity of 99 percent, a positive predictive value of 95 percent, a negative predictive value of 98 percent and an accuracy of 97 percent for the detection of fracture-associated edema.
Edema adjacent to the cortical endplates was better visualized using CaSupp index of 70 and 80, while extensive edema (BME extending throughout the trabecular bone compartment) was better visualized using a CaSupp index of 90 and 100 (chi2<0.0001).
No correlation between optimal CaSupp-index and trabecular density was found (p>0.2). CNR of BME increased with higher CaSupp index, with the peak value observed at the highest CaSupp-Index of 100.
Conclusion
CaSupp images reconstructed from IQon Elite Spectral CT allowed the clinician to better visualize and identify BME in traumatic fractured vertebrae with a high diagnostic accuracy using CaSupp indices of 70-100.
The high negative predictive value emphasizes the potential of CaSupp to rule out BME due to acute or subacute fracture. Additionally, CaSupp has shown the potential to assist the clinician in the detection of occult fractures since BME in vertebrae without morphologic signs of fracture in conventional CT were also detected.
Clinical Relevance
CaSupp results available on the IQon Elite Spectral CT can serve as a preset for reconstruction in patients with suspicion of BME.
Citation
Neuhaus, Victor, et al. “Bone Marrow Edema in Traumatic Vertebral Compression Fractures: Diagnostic Accuracy of Dual-Layer Detector CT Using Calcium Suppressed Images.” European Journal of Radiology, vol. 105, 2018, pp. 216–220., doi:10.1016/j.ejrad.2018.06.009.
Disclaimer: Results from case studies are not predictive of results in other cases. Results in other cases may vary.
Philips reserves the right to make changes in specifications and/or to discontinue any product at any time without notice or obligation and will not be liable for any consequences resulting from the use of this publication.
M&A Made Radiology What It Is Today
How Digital Radiography is Improving Outcomes for the Most Vulnerable Patients at Adventist Health White Memorial

Charles Ananian, M.D.

Adventist Health White Memorial in Los Angeles.
Whether it’s a premature baby or a critically ill child, treating little patients is a huge responsibility. After all, they can’t fend for themselves, placing them among the most vulnerable patient populations. Perhaps equally vulnerable are adult patients who face one of the most harrowing health prospects — the possibility of losing a limb to amputation.
That is why clinicians at Adventist Health White Memorial (AHWM) in East Los Angeles rely on Fujifilm’s digital radiography (DR) solutions to treat the most defenseless patients. Simply put, the right DR systems help AHWM’s clinical teams save both lives and limbs at the busy community hospital.
Pediatric Imaging: Safe, Accurate, Affordable
Digital X-rays are often a top priority in the diagnosis and treatment of young patients. The AHWM team depends on its FDR Go portable digital radiography system to attend to babies and children with speed and accuracy. It can be quickly deployed at the bedside, and digital images are acquired in minutes and available to clinicians for diagnosis within 10 minutes.
“When we have a critically ill child here that we are not able to bring down to radiology for an imaging study, [the] FDR Go allows us to do the X-ray at the bedside,” said Anthony Moretti, M.D., chairman of pediatrics, Adventist Health White Memorial. “It decreases costs, improves productivity and allows us to get a rapid diagnosis.”
For example, a common occurrence is when a child presents with increased work of breathing. While it could be just a cold, it could also be infection, or worse still, a life-threatening condition that requires air to be drained from the lungs. In these situations, said Moretti, the FDR Go provides a rapid diagnosis and can be a life-saving tool.
Premature babies often require chest X-rays, too. However, every time a tiny infant is moved, there are risks. Breathing tubes and central lines can be dislodged. With the FDR Go, nurses and technologists can do the imaging at the incubator.
“One of the greatest benefits of the FDR Go is that the infants get to stay in the unit. It’s minimal discomfort to the babies because all we have to do is slide the detector under them,” said Sarah Sarvi, NICU supervisor, Adventist Health White Memorial. “We don’t have to worry about extubating or about ventilating the patient from one floor to another. So it helps a lot and it also eases the tension of parents.”
What’s more, premature babies and sick children are susceptible to infections. So staying put is safer for the patient.
“We’re able to ensure the sterility of our practice because we’re not taking the babies down to areas where many sick adults have been,” said Sarvi. “We’re exposing them to less and less bacteria and potential nosocomial infections.”
Finally, keeping radiation dose to a minimum is top priority when imaging babies and children. FDR Go is compatible with all Fujifilm FDR D-EVO wireless detectors, which feature the manufacturer’s patented Irradiated Side Sampling (ISS) technology for exceptional image quality at low dose.
A fully integrated wireless system, the FDR Go is very durable, compact and easy-to-use. It was designed to fit into tight spaces while allowing users to easily manipulate the tube, making it ideal for the NICU, which typically houses a good deal of equipment.
Limb Preservation: DR Catches Complications, Reduces Amputations
Amputations cost the U.S. medical system billions of dollars every year. With the right technology, clinicians at AHWM’s Center for Limb Preservation are saving the organization money while saving patients’ limbs. The team sees on average 40 to 60 patients a day. The goal: catch complications early and avoid amputations.
The team has a dedicated FDR D-EVO Suite II on the premises. Charles Ananian, M.D., staff podiatrist, Center for Limb Preservation and Advanced Wound Care, Adventist Health White Memorial, said the sophisticated, world-class technology is a key contributor to the rapid diagnosis and treatment of patients and better overall outcomes.
“Normally what would happen is we send them into radiology maybe tomorrow and see them maybe the next day and by then, the problem could have compounded and become worse,” said Ananian. But with the FDR D-EVO Suite II onsite, “Now I see them at 4 o’clock, we send them right down the hall to get an X-ray here, I have the image in a few minutes, and I get to come up with a treatment plan for that patient in real time.”
The FDR D-EVO Suite II allows him to distinguish soft tissues from muscular skeletal parts, see bone abnormalities that are just beginning and view subtle changes in soft tissue that point to infection. This kind of image quality, said Ananian, is far superior to systems he has used just a couple of years ago and reduces errors by ensuring that he does not “miss something small.”
“With the FDR D-EVO Suite II, I get a quality image that’s highly efficient that I can manipulate with the software and really get a positive diagnosis that I feel comfortable with,” said Ananian.
The FDR D-EVO Suite II was also designed with speedy workflow in mind. The system allows techs to pre-set exam types and protocols before patients arrive. That, in turn, helps technologists avoid little mistakes and focus more on attending to the patient for a better and safer patient experience.
Finally, many patients at the Center for Limb Preservation are wheelchair-bound. The FDR D-EVO Suite’s II movable table makes for an easier, more efficient exam. The table elevates low to the patient’s comfortable seated level, making it easier to get on and off of the table, allowing techs to get images taken in roughly five to 10 minutes.
“Our FDR D-EVO Suite II technology has really changed the way we can impact peoples’ lives thanks to the image quality, the efficiency and the workflow benefits,” said Ananian. “It has truly sped up treatment and improved patients’ quality of life and outcomes by preventing amputations.”
For more information: www.fujimed.com
2018 - Year of the Patient
Researchers Awarded 2018 Canon Medical Systems USA/RSNA Research Grants

November 13, 2018 — The Radiological Society of North America (RSNA) Research & Education (R&E) Foundation recently announced the five recipients of its joint research grants with Canon Medical Systems USA for 2018. The 2018 Canon Medical Systems USA/RSNA Research Seed Grants were awarded to Pedram Heidari, M.D., Prashant Nagpal, M.D., and Adam Singer, M.D. The 2018 Canon Medical Systems USA Research Medical Student Grants went to Brandon Kenneth-Kouso Fields, BA, BM, and Anthony D. Yao, BS. These grants are made possible by Canon Medical Systems USA’s support of the RSNA R&E Foundation.
The Canon Medical Systems USA/RSNA Research Seed Grant provides $40,000 for a one-year project to test hypotheses and obtain pilot data in preparation for major grant applications:
- Pedram Heidari, M.D., Massachusetts General Hospital, will investigate a novel positron emission tomography (PET) probe for imaging of disease activity in inflammatory bowel disease (IBD) in mouse models. This PET probe is specific for granzyme B which is an important marker of T cell activation involved in pathogenies of IBD. Clinical translation of this imaging method, if successful, could potentially improve management and treatment of IBD;
- Prashant Nagpal, M.D., University of Iowa, with scientific advisor Mathews Jacob, Ph.D., will investigate whether 3-D self-navigated free-breathing cardiac magnetic resonance (CMR) sequences using manifold reconstruction algorithms compare well with the standard of care breath-held CMR sequences for patients with chronic obstructive pulmonary disease (COPD). If successful, this free-breathing CMR technique will allow high-quality comprehensive imaging in patients that cannot hold their breath; and
- Adam Singer, M.D., Emory University, will compare the performance of a novel sonographic scoring and reporting system to MRI for soft tissue sarcoma resection bed surveillance. If sonographic diagnostic accuracy is non-inferior to MRI, it could provide a more cost effective surveillance alternative particularly when MRI is contraindicated or degraded by artifacts.
The Canon Medical Systems USA/RSNA Research Medical Student Grant provides a $3,000 stipend, matched by the department for a total of $6,000 to pursue a research project in the radiologic sciences:
- Brandon Kenneth-Kouso “K.K.” Fields, BA, BM, University of Southern California, with scientific advisor George R. Matcuk Jr., M.D., will investigate the role of quantitative whole tumor volume MRI as a novel biomarker in evaluating response to neoadjuvant chemotherapy and radiation therapy in soft-tissue sarcomas. The principles developed with this project may lend new insight into early observable changes seen in this heterogeneous class of tumors to more accurately guide clinical management; and
- Anthony D. Yao, B.S., Rhode Island Hospital, with scientific advisor Ryan A. McTaggart, M.D., will investigate whether artificial intelligence can assist in the imaging of emergent large vessel occlusions (ELVOs) on computed tomography (CT)angiograms. If successful, neural networks may be implemented to aid radiologists in decreasing diagnostic time and improving patient outcomes.
The RSNA R&E Foundation Board of Trustees approved funding for $4 million in radiology research and education grants this year, achieving a funding rate of 35 percent of grant applicants. “The R&E Foundation is grateful for Canon Medical Systems USA’s support of the 2018 grant recipients. This longstanding partnership and commitment is a vital component in ensuring research innovation and seeding the future of radiology,” said N. Reed Dunnick, M.D., chair of the R&E Foundation Board of Trustees.
For more information: www.rsna.org/foundation, www.us.medical.canon
BLOG: How Advanced X-Ray Systems Help Meet Outpatient Challenges
How to Market Healthcare Artificial Intelligence Software

Artificial intelligence was the hottest topic at the 2018 Radiological Society of North America (RSNA)) meeting, which included a large area with its own presentation therater set asside for AI vendors.
Hands down, the hottest topic in radiology the past two years has been the implementation of artificial intelligence (AI) in radiology and how it will be integrated into medical imaging. As products now begin gaining U.S. Food and Drug Administration (FDA) market clearance, the next question with this potential technology revolution is how exactly to integrate the scores of new software applications from a large number of vendors into daily practice.
The majority of the new AI software is coming from small startup companies and each piece of software cleared by the FDA covers one very specific medical imaging diagnostic review. Radiology experts have started asking how AI technology will be viable if it requires hundreds of contracts and integration of a large number of disparate software programs into the hospital or enterprise imaging system PACS. They say the need to deal with a large number of small companies for various pieces of the AI puzzle would be a nightmare for legal review, contracts and hospital IT departments.
At the 2018 Radiological Society of North America (RSNA) meeting, about 150 companies showed technology that integrates some level of AI or deep learning. Many were concentrated in the specific Machine Learning Showcase area. However, only a small handful of these vendors actually have an FDA cleared project. But, this is changing rapidly and the number of new AI applications for medical imaging is expected to explode the next couple years. While these technologies may offer improved outcomes by immediately differentiating between a hemorrhagic and ischemic stroke, or identify a pneumothorax during a bedside X-ray, there are reservations in the market as to how this technology from numerous vendors can logically be implemented.
The Creation of AI App Stores
Taking a note from Apple's App Store, larger healthcare IT vendors are starting to partner with smaller companies to provide a combination of home-grown and third-party apps through a web-based AI app store platform. Partnering companies need to meet compatibility and interface requirements that match those of the primary vendor's products to allow plug-and-play use. This model allows hospitals a single location and vendor to purchase AI software that offer a common IT interface.
Elements of these platforms started to appear in early 2017, with the introduction of Siemen's Digital Ecosystem. That platform offers an online menu of apps from Siemens and partner IT firms, including some that offer AI enabled technology. At RSNA 2018, numerous companies announced the creation of, or expanded capabilities of, AI app stores. Examples include TeraRecon's Envoy AI, and AI app stores offered by Blackford, Visage, Sectra and IBM Watson.
IBM Watson announcing at RSNA 2018 it is starting to partner with various AI vendors to offer their products on its new AI Marketplace. This was an interesting revelation, because IBM Watson was previously perceived to be the 500-pound gorilla in the medical AI space. The company originally planned to start rolling out a series of its own, in-house developed AI solutions across the healthcare spectrum. However, the company faced set backs like MD Anderson ending its partnership on cancer imaging AI, and the slow progress in commercializing AI products while many small startups began getting FDA clearances for AI clinical review products. In a booth presentation at RSNA, Steve Tolle, VP, global strategy and business development, acknowledged a single vendor cannot yet be all things to all people in the AI space. In order to be a major player in the growing AI market, he said the company needed to partner to offer access to a wide variety of AI apps.
Tolle said IBM Watson's AI Marketplace will offer standardized application programming interfaces (API) for building or integrating third party software with availability through the IBM Cloud.
GE Healthcare at RSNA unveiled its Edison platform, which is designed to help accelerate the development and adoption of new technologies, especially AI. GE’s clinical partners would use the newly branded platform to develop algorithms and speed the path of advances in data processing to Edison applications and smart devices.
Where is AI Being Implemented in Medical Imaging?
There are four main areas where AI is being implemented:
1. Computer-aided diagnosis
2. Clinical decision support
3. Quantitative analysis tools
4. Computer-aided detection
Automated quantification tools are entering a level of maturity and acceptance in the market, with AI making measurements from imaging exams and auto filling fields or performing calculations that were previously manual and time consuming. However, new frontiers in AI-driven auto quantification tools include radiomics, imaging biomarkers and virtual biopsies.
AI-drive quantitative analysis tools also are being used in data analytics software used for departmental and hospital business management. Rather than the old and cumbersome process of running Crystal reports or manually tabulating data points, AI software can data mine connected electronic medical records, billing systems, patient scheduling and even individual scanning equipment. This data can be mined for everything from the amount of X-ray dose used by specific technologists or machines for specific exam protocols, to predictive analytics software that can pin point which days and times there were be back ups in the radiology department when additional staff should be scheduled.
The newer AI areas of computer-aided diagnosis and clinical decision support were only recently introduced into the market and may take several years before they are found in general use. The primary areas where AI image diagnostic software is being developed and commercialized is for critical findings such as stroke or other maladies where timing is crucial. Other areas include identification of incidental findings and tools to reduce the time it takes to review complex exams, or to help auto triage patients who need additional or more immediate care.
Computer-aided detection has been around for years, but with the addition of machine learning algorithms, experts in the field are calling the newer generation AI-supported software "CAD that works," because of its much lower rate of false positives.
Lots of Data is Needed to Validate AI
A big question in AI, especially with diagnostic algorithms, is how it gets validated. Large amounts of de-identifed data is needed to train deep learning software. At RSNA 2018, Philips Healthcare unveiled its IntelliSpace Discovery 3.0 platform to help facilitate the AI training data required. It is an advanced visualization and analysis platform designed specifically to support imaging research, and an extended version of its IntelliSpace Enterprise Edition that includes Philips’ PerformanceBridge. Providers at dozens of institutions are already using the Discovery 3.0 version to prepare patient data to train and validate deep learning algorithms.
Related Artificial Intelligence Content:
VIDEO: RSNA Post-game Report on Artificial Intelligence — ITN editors Dave Fornell and Greg Freiherr discuss the AI trends they saw at RSNA 2018
How Artificial Intelligence Will Change Medical Imaging
VIDEO: AI, Analytics and Informatics: The Future is Here— Interview with RSNA 2017 keynote speaker Michael Recht, M.D.
VIDEO: A Walk Through the RSNA 2018 Machine Learning Showcase
Achieving Optimum Reject Rate in Digital Radiography

In today’s digital environment, a radiologist only sees images saved and shared to the PACS, so a firm understanding of X-ray reject rates is crucial for high image quality and good workflow.
X-rays were the first medical imaging technology to be invented, and they remain one of the most commonly performed exams worldwide today. The technology has evolved immensely since it was first discovered in 1895, progressing from the original screen film to computed radiography (CR) to today’s digital radiography (DR) units. While the technology has changed, the need for high-quality images at the lowest possible dose to the patient has not, and has even heightened as medicine shifts toward value-based care. For DR, this means optimizing protocols and techniques through a robust system of reject rate analysis.
Defining Rejects and Reject Analysis
Before the need to retake an X-ray can be determined, it must be decided what constitutes a “rejected” image. According to Ingrid Reiser, Ph.D., DABR, a clinical diagnostic physicist and associate professor of radiology at the University of Chicago, rejects are patient images that are discarded by the technologist without being presented to the radiologist. She shared the definition as part of a presentation on reject rate analysis at the 2018 Radiological Society of North America (RSNA) annual meeting.
In today’s digital environment, the second part of that definition — without being presented to the radiologist — is the key consideration. A radiologist will only see images that are saved and shared to the picture archiving and communication system (PACS), so they likely have no idea how many images are actually acquired during an exam. In this scenario, having a firm understanding of reject rates is crucial for maintaining high image quality and good workflow. Furthermore, Reiser noted, each image not sent to the radiologist’s workstation represents wasted radiation dose to the patient — a cardinal sin in radiology.
Reiser stressed, however, that the goal of reject rate analysis is not to reduce the rate to zero. Poor-quality images are an unavoidable part of diagnostic imaging, and if they are determined to be poor they should be eliminated from consideration.
Digital Challenges of Reject Analysis
Examining rejects and reject rates tells a story, according to Alisa Walz-Flannigan, Ph.D., diagnostic medical physicist at the Mayo Clinic in Rochester, Minn. Reviewing rejected images (and rejected image data) provides information about image quality standards, inherent imaging problems and modes of failure, as well as how technologists troubleshoot these types of issues.
While technological advances have eliminated many of the challenges of screen-film and computed radiography, digital X-rays come with their own set of difficulties:
- Many hospitals and imaging centers use imaging systems from multiple vendors, and each vendor may collect different bits of data using different methods;
- As a result, information retrieval may be cumbersome, and some desired information may not be retrievable at all;
- Reject analysis may require a software add-on at additional cost; or
- The reject analysis software could interfere with clinical operation.
Quality Control for DR
While imaging standards and practices are unique to every radiology department, there are resources available for practitioners seeking guidance. The American Association of Physicists in Medicine (AAPM) Task Group 151 published a report in 2015 on quality control procedures in digital radiography, with the goal of recommending consistency tests for optimum image acquisition. The task group said the tests should be performed by a medical physicist, or a radiology technologist under the supervision of a medical physicist.
One of the main procedures recommended by the task group is defining a fault tree of actions that need to be taken when certain fault conditions are met. The diagram should begin with a particular problem, such as an artifact in a patient image, and run through possible corrective actions based on the source and severity of the artifact.
The AAPM report also reinforces the importance of performing reject rate analysis: The task force cites a prior study of 18 radiology departments where 14 percent of patient exposure in projection radiography was deemed to be the result of repeated images.
Ultimately, the task group recommends a target reject rate of 8 percent for DR units. A literature review found reject rates for screen-film radiography hovered around 10 percent, with nearly half of all rejects due to exposure errors. The task force arrived at the 8 percent figure for digital with the assumption that exposure errors will be fewer with DR. Ten percent is the recommended threshold for further investigation and possible corrective action.
Starting a Reject Rate Analysis Project
While the principles of reject rate analysis may be straightforward, starting a Reject Rate Analysis Project (RRA) is a major undertaking. Reiser shared her experience leading an RRA project for digital X-ray at the University of Chicago beginning in 2014.
Reiser acknowledged that prior to 2014, all reject rates were self-reported by technologists. The reasons for this were twofold and largely practical: Reject analysis software was optional for many of the department’s DR units, and the features were turned off for systems that were capable of RRA. This was done, Reiser said, to avoid file storage problems with the limited hard drive space.
Quarterly reviews were conducted for each X-ray tech’s workload, and each tech was assigned a quality score for eight randomly chosen exams. (Approximately three exams were chosen per month to ensure a wide range of images were reviewed.) An in-person meeting was conducted with the technologist and the imaging technology coordinator or manager to discuss the findings. Those discussions included:
- Review of the technologist’s reject rate by category (anatomy, clinical area, artifacts, motion, etc.);
- Review of any quality improvement (QI) tickets issued and tracked through the PACS by radiologists. Tickets are assigned by improvement category, such as Joint Commission patient safety goals or image quality reasons. Reiser said the QI tickets were kept as an ongoing quality review measure;
- Changes in reject rates; and
- Ideas or feedback for future quality improvements.
Any corrective actions, if required, are also identified during the in-person meetings.
Designing Interventions
Reiser concluded by offering advice for department heads looking to design and implement their own reject rate analysis interventions.
At the University of Chicago, the radiology department conducts a series of in-service meetings to teach the specific elements of reject analysis. The meetings cover classification of reject categories, a review of current reject rates and discussion of when to reject an image.
To focus on image review, separate in-services are conducted for technologists targeting specific exam types. Example focus areas include portable X-ray exams, wrist imaging, chest X-rays and lumbar spine exams. One rejected image is chosen randomly for each rejection category and compared with the “approved” diagnostic image.
The goal of these meetings, according to Reiser, is to develop image critique skills as a group, and encourage ownership and accountability for exams performed. For this reason, she recommended using the names of the technologists attached to each image set during discussions, rather than coding for usernames.
Walz-Flannigan shared key questions in her RSNA presentation that should be considered during image review:
- Is the accumulated data accurate?
- Are standards being followed (with the system setup and technologist use)?
- Do standards need to be adjusted?
- Are good images being rejected?
- What challenges are techs facing?
Reiser concluded that while interventions may not always lead to better reject rates, they may still improve image quality, making them a critical component of patient care.
Most Popular Imaging Technology Content in January 2019

The top article from January was about researchers in Sweden using computed tomography (CT) to image the soft tissue of an ancient Egyptian mummy’s hand down to a microscopic level. Non-destructive imaging of human and animal mummies with X-rays and CT has been a boon to the fields of archaeology and paleopathology.
February 1, 2019 — Here is the list of the most popular content on the Imaging Technology News (ITN) magazine website from the month of January 2019. This is based on website’s 200,363 pageviews for the month:
1. CT Technique Expands Possibilities of Imaging Ancient Remains
2. First Arterial and Venous Atlas of the Human Brain Released
3. Machine Learning Uncovers New Insights Into Human Brain Through fMRI
4. FDA Clears United Imaging Healthcare uExplorer Total-Body Scanner
5. How to Market Healthcare Artificial Intelligence Software
6. New Pathology Guideline Advances Accuracy in Breast Cancer Testing
7. Brachytherapy Alone Superior Treatment for Intermediate-Risk Prostate Cancer
8. Electronic Brachytherapy Effective in Long-Term Study of 1,000 Early-Stage Breast Cancers
9. Shimadzu Medical Systems USA Acquires Core Medical Imaging
10. Artificial Intelligence Pinpoints Nine Different Abnormalities in Head Scans
11. Artificial Intelligence Used in Clinical Practice to Measure Breast Density
12. Novel Technique May Significantly Reduce Breast Biopsies
13. Digital Mammography Increases Breast Cancer Detection
14. Southern Asia's First Proton Therapy Center Begins Treatments
15. VIDEO: Researchers Use MRI to Predict Alzheimer's Disease — Interview with Cyrus A. Raji, M.D.,
16. VIDEO: AI in Tumor Diagnostics, Treatment and Follow-up — Intercview with Julius Chapiro, M.D.
17. Artificial Intelligence Shows Potential for Triaging Chest X-rays
18. Achieving Optimum Reject Rate in Digital Radiography
19. VIDEO: Personalizing Breast Care with Invenia ABUS 2.0
20. Philips Launches Azurion With FlexArm Angiography System
Hear and Now: How to Boost Cybersecurity in Medical Imaging


Cyber hackers pose a worsening threat to radiology and the rest of medical imaging. This risk might be reduced, however, by applying best practices developed by professional organizations such as HIMSS (Healthcare Information and Management Systems Society) and government agencies such as HHS (Health and Human Services) and NIST (National Institute of Standards).
This help is coming none too soon, according to Axel Wirth, distinguished technical architect at Symantec and one of the world’s foremost authorities on cybersecurity.
“Attacks are not only becoming more frequent and more sophisticated but they are also becoming much more malicious and purposeful,” said Wirth in an ITN podcast on cybersecurity in medical imaging and health care.
Wirth, who has spoken often on health IT topics, including medical device cyber safety, will deliver the keynote speech February 11 at the Cybersecurity forum during the annual HIMSS convention in Orlando.
Why Hackers Target Medical Imaging
Medical imaging presents easy targets to attackers. This is one of the prime reasons it has been attacked, Wirth said during ITN’s podcast on cybersecurity. Scanners “and things like PACS and PACS workstations were exploited by attackers as a beachhead,” Wirth said in the podcast, noting that cyber attacks on imaging equipment and imaging networks have been done to access “higher valued information.”
But cyber attackers may also target medical imaging and health care for other reasons, he said. Nation states and nation-sponsored groups may be after information about the design of medical devices and formularies for new pharmaceuticals, as well as clinical trial data.
Or they may use malware to encrypt patient data, and then offer to sell the decryption code to victims. Health care is not the only industry susceptible to ransomware, but it is especially vulnerable. Wirth said, “I can’t tell a patient that arrives in the emergency room with chest pain that unfortunately we are dealing with a cyber attack: ‘Can you come back next week?’ ”
The good news is that cybersecurity in health care is maturing “at a fairly rapid pace,” Wirth said in the podcast. The key to continuing that pace is making sure cybersecurity is applied throughout health care — “not just (to) a few select thought leaders and large hospitals.”
Where To Find Best Practices in Cybersecurity
An HHS document detailing cybersecurity practices in health care notes the cybersecurity risks and how different size practices might address them. “What is good about this document is that they really broke it down, based on size … and maturity of the organization,” said Wirth, who cited similarly crafted HIMSS guidances“for various types of organizations based on size and maturity level.”
Wirth also cited a NIST publication that establishes a generic cybersecurity framework. “Different industries and individual organizations (can) take that framework, adapt it to their needs and circumstances, and then develop a roadmap for a path to security,” he said.
As the interconnectivity of medical imaging increases due to cloud computing, analytics and data storage, as well as the expansion of enterprise imaging, the threat from cyber criminals is growing. “More connectivity always creates more opportunity (for cyber attackers),” Wirth said.
In the podcast, Wirth describes steps that providers might take in concert with equipment manufacturers to counter this rising threat. Some “would depend on the type of equipment you are purchasing,” he said. In the podcast, he contrasts how considerations differ when purchasing ultrasound scanners, for example, versus PET/CTs.
Additional Reading:
FDA and DHS Expand Partnership on Medical Device Cybersecurity (https://www.itnonline.com/content/fda-and-dhs-expand-partnership-medical-device-cybersecurity )
How To Stop (Or Slow) Hackers (https://www.itnonline.com/content/blogs/greg-freiherr-industry-consultant/how-stop-or-slow-hackers )
Agents of Change: Cybersecurity In A World Of Old And New (https://www.itnonline.com/content/blogs/greg-freiherr-industry-consultant/agents-change-cybersecurity-world-old-and-new )
Greg Freiherr is a contributing editor for Imaging Technology News (ITN). Over the past three decades, Freiherr has served as business and technology editor for publications in medical imaging, as well as consulted for vendors, professional organizations, academia and financial institutions.
Editor's note: In preparation for the upcoming HIMSS (Healthcare Information and Management Systems Society) Conference on Feb. 11, contributing editor Greg Freiherr begins the show coverage with this exclusive podcast and accompanying blog. This is the first podcast in a series of three.
How to Boost Cybersecurity in Medical Imaging
5 Key Trends in New Ultrasound Technology

An example of Philips' TrueVue technology, which offers photo-realistic rendering and the ability to change the location of the lighting source on 3-D ultrasound images. In this example of two Amplazer transcatheter septal occluder devices in the heart, the operator demonstrating the product was able to push the lighting source behind the devices into the other chamber of the heart. This illuminated a hole that was still present that the occluders did not seal.
Here is a list of six key trends in ultrasound technology that Imaging Technology News (ITN) has seen at radiology and echocardiography conferences over the past two years. For hospitals looking at purchasing new imaging systems, this list will be helpful when comparing vendors.
1. Improving Ultrasound Workflow
In an era of lower reimbursements and a healthcare reform environment where providers are being asked to become more productive, increasing patient throughput without sacrificing quality has become a major topic. On ultrasound imaging systems and their connected reporting systems, this means streamlining the workflow process. Newer generation ultrasound systems offer features like fewer dropdown menus, less keystrokes, faster processing times and the automation or semi-automation of measurements. Here are some examples from recently released imaging systems.
A new version of the Canon Aplio 900 CV system released in 2018 was designed with 40 percent fewer keys to simplify workflow. It also is 50 percent lighter than previous Toshiba echo systems.
Hitachi launched its new Arietta 65 mid-range ultrasound system at the Radiological Society of North America (RSNA) 2018 meeting. It uses a smaller track ball to make the keyboard smaller and enable shorter hand movements, making the system more ergonomic.
The Samsung RS85 ultrasound system received FDA clearance in 2018 and was designed so many multi-step actions are now combined into a single step to reduce keystrokes and repetitive user interactions.
Another example is Konica Minolta's new Sonimage HS1, which offers simplified one-button image optimization. Multiple imaging parameters, such as frequency, focus and compounding, change automatically when adjusting the depth.
Automated Lesion Segmentation was introduced on the GE Healthcare Logiq E10 system at RSNA 2018 to increase productivity through automation. There are significant ergonomic challenges due to repetitive exam steps. This software helps eliminate the need for the user to measure lesions manually, by segmenting an identified breast, thyroid or liver lesion and automatically providing a trace of the lesion and corresponding area. This feature also helps ensure consistency among different users, or even the same user, for documentation and follow-ups.
2. Integration of Artificial Intelligence Into Ultrasound
Automation of time-consuming tasks, quantification and picking out the ideal image slice from a 3-D dataset is starting to be performed by artificial intelligence (AI). Many high-end ultrasound systems already integrate some level of AI and most new systems on all tiers moving forward will likely integrate increasing levels of AI.
Integrating AI algorithms in the backend of ultrasound systems began a few years ago with the goal of speeding workflows. Elements of this are built into Siemens' eSie Flow valve analysis software for 3-D heart valve assessments. Philips' Epiq system uses anatomical intelligence, where the AI can automatically identify, segment and color code the anatomy in the scanning field. It also can select the optimal scanning slice view for various exams, extracting it from 3-D datasets, improving reproducibility regardless of the sonographers level of experience. The Philips Epiq and Affiniti ultrasound systems featured at RSNA 2018 offer anatomical intelligence for breast imaging to enhance reproducibility and streamline workflow. The automation and AI provides visual mapping and annotation of screened anatomy, with minimal user interaction.
"In a standard echo study, a sonographer will acquire hundreds of images. If a cardiologist wants to look at images from specific views, they need to review each individual clip – almost like flipping through a dense text book without a table of contents," said Al Lojewski, the general manager of GE Healthcare, cardiovascular ultrasound division. "AI brings the potential for the cardiologist to indicate the specific structure so that the system may automatically pull all the images with that view, or identify specific anatomy and function of the heart bringing the exam review process in line with the clinician’s diagnostic questions – rather than managing a vast collection of images and measurements. This could save them critical time that they can now spend with their patients."
The newest version of the Konica Minolta Sonimage HS1 uses AI-voice recognition controls for hands-free operation. Aimed at musculoskeletal (MSK) interventional procedures, it has the ability to control system functions through simple voice commands. The clinician can hold the transducer in one hand and a needle or syringe in the other, eliminating the need for an assistant and maintaining the sterile field during procedures.
To enhance the functionality of its portable Vscan Extend handheld, pocket-sized ultrasound, GE Healthcare added the DiA Imaging Analysis AI-powered LVivo EF for automated ejection fraction (EF) measurements. Traditionally, most EF interpretation at the point of care (POC) is conducted through visual estimation, with clinician experience levels varying across POC settings. LVivo EF addresses this challenge by quickly and efficiently providing clinicians with left ventricle EF scoring and volume measurements using AI and advanced pattern recognition algorithms.
Northwestern Medicine recently began a study using Bay Labs’ AI-based EchoGPS cardiac ultrasound guidance software to enable certified medical assistants (CMAs) with no prior scanning experience to capture high-quality echocardiograms. The study will also evaluate the use of its EchoMD measurement and interpretation software suite to detect certain types of heart disease among patients 65 years and older undergoing routine physical examinations in primary care settings. The SHAPE (Seeing the Heart with AI Powered Echo) study is the first to evaluate AI-guided ultrasound acquisition by CMAs. It will enroll 1,200 patients.
“Deep learning will have a profound impact on cardiac imaging in the future, and the ability to simplify acquisition will be a tremendous advance to bring echocardiograms to the point-of-care in primary care offices,” said Patrick M. McCarthy, M.D., chief of cardiac surgery at Northwestern Memorial Hospital, executive director, Northwestern Medicine Bluhm Cardiovascular Institute and principal investigator on the project.
3. Advancements in 3-D Ultrasound
The slower frame rates and larger expense of 3-D ultrasound has limited its wider adoption, but its application is some specialty areas has helped rapidly expand therapies such as transcatheter structural heart interventions. The use of 3-D has big applications when the imaging is used by specialists for procedural planning or guidance, where the 3-D can offer a "surgeon's view" of the anatomy. The technology is also used to help guide catheter procedures in complex anatomy.
"The technology just keeps getting better and better in regards to 3-D ultrasound," said Sunil Mankad, M.D., FASE, director of transesophageal echocardiography (TEE) at Mayo Clinic, Rochester, Minn. "In cardiology, what this allows you to see the relationships with underlying structures. I don't think we are ready to put 2-D echo to bed yet, because usually you need a combination of the two. The frame rate is currently far better with 2-D over 3-D, but 3-D is critical for things like structural heart evaluations."
He said all the vendors are getting better with increasing frame rates, better resolution and improved color Doppler. Mankad said the trend is definitely headed toward 3-D taking over 2-D market share in the years to come.
Most 3-D systems are still operating below 30 frames per second, but the technology and speed is improving each year, said Lissa Sugeng, M.D., associate professor of medicine, director of echocardiography and director of the Yale Echo Core Lab, Yale School of Medicine. She believes the time has come where all echo labs need at least one 3-D echo system. She said these systems are needed minimally for cardio-oncology patient assessments. The imaging they provide is also valuable for surgeons and structural heart interventionalists who need the 3-D imaging for a more comprehensive assessment and visualization of valves, septal defects and the left atrial appendage.
She said some vendors have attempted to increase their volume rates by using multi-beat acquisitions, but Sugeng said she would prefer a one-beat solution to avoid image-stitching artifacts.
As computing power continues to advance, Sugeng said it is inevitable that frame rates will catch up in 3-D systems. "I think all the companies are trying to strive for that," she explained.
In 2018, GE Healthcare released its Imaging Elevated release of its cSound image reconstruction technology. The technology aids imaging quality, workflow and quantification on the Vivid E95 cardiac imaging system. It leverages GPU processing to improve the volume frame rate, which GE refers to as volume max, or Vmax. This allows for nearly triple the frame rate speeds for TEE in a single beat over previous generation systems.
Watch a VIDEO interview with Sugeng.
Watch a VIDEO interview with Mankad.
4. New Ultrasound Visualization Methods
Vendors have moved beyond basic 2-D and 3-D imaging to offer new ways to reconstruct images to speed evaluations and make them easier to understand.
A good example of new imaging unveiled at RSNA 2018 was developed to address fetal heart and brain imaging. Detailed fetal cardiac assessments are difficult to perform because of the small size and extremely fast heart rates. At 18 weeks, the fetal heart is the size of an olive and beating about 150 times per minute. Additionally, the structure itself is extremely complex and with the baby in constant motion, it is always a moving target. Imaging is important, because congenital heart defects affect one out of every 110 babies born globally.
GE Healthcare's fetalHQ heart and vascular analysis software for fetal ultrasound. Offered on the Voluson E10, it helps evaluate the fetal heart shape, size and contractibility in less than three minutes. A feature called Radiant Flow shows the blood flow in a 3-D view. It can also help show slow-flow blood, such as neurovascular circulation.
Watch a VIDEO example of this technology showing fetal cardiac blood flow and cerebral blood flow.
Another example released in 2018 is Philips' TrueVue, which offers photo-realistic rendering and the ability to change the location of the lighting source on 3-D ultrasound images. It allows users to changing the lighting conditions to improve contrast. The light source can also be moved around to change the shadows and add more depth perception. The light source also can be pushed through the tissue to back light the anatomical structures,
Watch the VIDEO: Photo-realistic Lighting to Enhance 3D Echocardiography.
Imaging blood flow in small, slow flow vessels was not possible with ultrasound prior to a couple years ago, but a few vendors now offer systems with this ability. The feature provides an additional way to check lesions for indications of cancer or inflammation. One of the first was the Canon Apollo 900 CV, which can show blood flow in capillary vessels. Hitachi's Arietta 65 mid-range system offers a feature to visualize small blood vessels to better view perfusion in organs like the kidney. The Samsung RS85 also offers MV-Flow to visualize slow-flow micro vascularized structures.
The Canon Apollo 900 CV system also launched in 2018 a new way to visualize the heart with echo called quad-chamber tracking. It tracks the blood volumes for all four chambers in a single, 3-D view. It offers both end diastolic and end systolic views of the chambers. This allows a picture of the entire heart function at once, rather than looking at one chamber at a time.
See a VIDEO demonstration of this technology included in this collection of new technologies.
Baptist Health South Florida became the first commercial install site last fall for the Siemens Healthineers' Acuson Sequoia. The system will help enhance imaging capabilities for gastroenterology, primary care and bariatric specialties. The Sequoia enables high-resolution imaging that automatically adapts to patients’ size and personal physical characteristics, contributing to more confident diagnosis. It adapts to the patient's bioacoustic variations in tissue density, stiffness and ultrasound beam absorption. This allows the system to penetration up to 40 cm without image quality degradation often caused by attenuating echo signals.
5. Point-of-care Ultrasound
The past few years there has been an explosion in the use of POC ultrasound (POCUS). Vendors have released numerous small pocket sized, or slightly larger cart or wall mounted, basic ultrasound systems to allow a quick look inside the patient for more accurate and faster assessments, or to determine if a higher level of imaging is needed. POCUS has moved into many subspecialties, most notably in emergency medicine, critical care, internal medicine and anesthesia.
"Some institutions use full ultrasound systems to do all of their point-of-care ultrasound. There are many tools for different jobs, and the problem is that many people have different jobs they need to do. Some centers doing very sophisticated measurements will want a full system. Some people say they need the portability because they go all over the hospital to do bedside ultrasounds and use these systems like a stethoscope. A person like that might want a hand-held device," explained Michael Lanspa, M.D., director of critical care echocardiography services, Intermountain Medical Center, Salt Lake City, Utah. He is involved in new POCUS training initiatives by the American Society of Echocardiography (ASE) to fill the void where doctors from various subspecialties are looking for basic ultrasound training Lanspa also serves on the educational committees for ASE, the American Thoracic Society (ATS) and the Society of Critical Care Medicine.
He said in 2019, the National Board of Echo will offer a formal certification for critical care ultrasound, and ASE will be offering a review course for it.
There have been several dedicated POCUS systems introduced in the past couple years. Fujifilm SonoSite Inc. presented a complete portfolio of POCUS systems at RSNA 2018. Its SonoSite X-Porte portable kiosk system combines touch screen controls and a customizable interface that offers more than 80 real-time educational visual guides and tutorials. The SonoSite Edge II clamshell system offers an intuitive interface for easier access to frequently used functions and a wide-angle display with an anti-reflection coating for minimal adjustments during viewing. The SonoSite SII uses a simple portrait display and smart user interface. The SonoSite iViz fits in the palm of the hand, providing quick answers in clinical questions.
See a VIDEO example SonoSite iViz system.
In 2018, Healcerion gained FDA clearance for its Sonon 300L wireless handheld ultrasound device that works with a table device to display the images. It provides a flexible imaging device at less than 1/10 the cost of a traditional ultrasound machine, with a user interface anyone can learn in minutes, according to the company.
In March 2018, Philips announced the first integrated tele-ultrasound solution on Philips’ Lumify portable ultrasound system. The system is composed of a transducer paired with a mobile device and an app to convert smart phones or tables into an ultrasound system. The new feature connects clinicians around the globe in real time by turning a compatible smart device into an integrated tele-ultrasound solution, combining two-way audio-visual calls with live ultrasound streaming. Users can have a live, face-to-face conversation on their Lumify ultrasound system. Users can switch to the front-facing camera on their smart device to show the position of the probe. They can then share the Lumify image stream, so both parties are simultaneously viewing the live ultrasound image and probe positioning.
Another example of POCUS moving into breast imaging is the 2018 release of Hologic’s new Viera portable breast ultrasound system. The wireless ultrasound scanner provides physicians with the opportunity for earlier diagnoses and an optimized clinical workflow. It can guide interventional procedures such as biopsies, marker placements and wire localizations. It also can transmit breast images to smart devices or PACS.
One of the most versatile POCUS systems recently introduced is the Butterfly IQ ultrasound transducer and app. The company displayed at RSNA for the first time in 2018, and showed attendees how they can convert their iPhones into a reasonably good quality diagnostic ultrasound system. Its FDA-cleared technology is one of the first “ultrasound system on a chip” to be released commercially. It consists of a transducer that connects to an iPhone or iPad to record ultrasounds.
The system has 18 different applications for specialized images, including cardiac imaging, vascular, aorta, lung, abdominal and others. The apps allow for quantification and offer features usually found only on larger cart-based systems. The company said 90 percent of the work that can be done on a cart-based system can be performed with the Butterfly IQ. The apps allow basic measurements and annotations, and images can be transferred from the device into a cloud server for storage or transferring to a picture archiving and communication system (PACS) as a DICOM format. The images also can be saved as a PNG or videos in the MP4 format.
The ultrasound system on a chip technology allows a single transducer to be reconfigured with a simple setting to image as a curved, phased or linear probe, rather than needing to physically have and swap out various transducers. The system also allows for changes on the fly for beam sharpness, frequency and focal depth. The system also offers there scanning modes — M-mode, B-mode and color Doppler. The 2-D Butterfly transducer is composed of 9,000 elements and does not use traditional Piezo crystal technology.
Leveraging the fact that the platform operates on a smartphone, users can tag colleagues on to images they create to get quick consultations in or outside of the hospital. The tags create a de-identified image link so there is no violation of HIPAA privacy regulations.
Related Ultrasound Content:
Latest Ultrasound Advances on Display at RSNA 2018
Recent Advances in Echocardiography Technology
Top Technology Trends in Echocardiography at ASE 2018
VIDEO: Editor’s Choice of the Most Innovative Echo Technology at ASE 2018
4 Trends in Interventional Lab Angiography Systems

Image courtesy of Philips
There are several recent trends in X-ray angiography imaging systems that hospitals should be aware of if they are looking for new or replacement technology.
1. Technology to Lower Radiation Dose
As interventional procedures become more complex, imaging and procedural times naturally increase. To compensate for the higher imaging X-ray doses involved, vendors have developed a new generation of angiography systems that address the need to lower dose for both the operator and the patients. The current-generation systems offered by Canon, GE Healthcare, Philips, Shimadzu and Siemens all offer lower X-ray dose while preserving image quality. This has been accomplished with a combination of new X-ray tubes, more sensitive detectors, new image reconstruction software, image hold technologies, and by using better navigation and multimodality image fusion software to guide procedures.
The most recent example of this was in January 2019, with Philips launching its Azurion 7 with FlexArm system. It is designed to enhance positioning flexibility for image-guided procedures. Due to increasingly complex interventions, clinicians need to quickly and easily visualize critical anatomy and identify changes to the patient during the procedure. This system includes technology to make this easier in both 2-D and 3-D. As the clinician moves the system, the image beam automatically maintains alignment with the patient.
The Azurion with FlexArm’s design provides a high level of flexibility with movement on eight different axes, all controlled with its single controller. Simulation tests with clinicians demonstrated the potential to significantly reduce the repositioning of the patient, staff and equipment to improve access, including radial access. The system has both CE mark and U.S. Food and Drug Administration (FDA) clearance.
2. Hemo Integration
When a hospital installs a new interventional lab, many want a complete package so they do not have to subcontract with multiple vendors. A key element of the labs is integration with a hemodynamic system. All the major angiography vendors now partner with other vendors to offer complete solutions with hemodynamics.
The most recent partnership was Shimadzu Medical Systems USA and Change Healthcare partnering last fall, where Shimadzu will offer Change’s Cardiology Hemo on its Trinias line of angiography systems. Trinias is a single-plane system available in floor or ceiling mounts or as a bi-plane mounted system.
3. Echo Fusion Solutions
Complex procedures, especially in the structural heart space, require visualization of the surrounding soft-tissue anatomy beyond what 2-D angiography can offer. Ultrasound is usually employed for complementary imaging during procedures, usually with transesophageal echo (TEE). A couple vendors have taken TEE a step further by integrating with the live fluoro imaging to co-register the images and display them in one view.
There were two new product introductions in this space in 2018. Philips introduced the Epiq CVxi interventional cardiovascular ultrasound system. Specifically designed for use in the cath lab, the Epiq CVxi with EchoNavigator streamlines communication between the interventional cardiologist and the echocardiographer. Combining live ultrasound and X-ray information into one fused view, it helps interventional cardiologists oversee procedures along with the location of key anatomical structures. The system uses Epiq’s artificial intelligence to automatically identify and extract anatomical structures to speed the image fusion process and show anatomical outlines on live TEE or fluoroscopy.
Siemens introduced its version of a TEE/angiography fusion system with its TrueFusion, released in 2018. It allows fusion of TEE, cardiac computed tomography angiography (CTA) and live fluoro to aid navigation in complex heart procedures.
4. Integration of Augmented Reality
A new technology that is already on the horizon to aid procedural navigation in the cath lab is augmented reality (AR). The technology will enable operators to see true 3-D images of anatomy in a heads-up display while they are looking at the patient or at the main screen in the lab.
Philips Healthcare showed a conceptual work-in-progress of this technology at the Radiological Society of North America (RSNA) meeting in 2017, allowing attendees to put on the headset and manipulate 3-D images with hand movements in the air. NovaRad and TeraRecon have already commercialized similar AR technology to aid in advanced visualization of patient 3-D datasets. Novarad’s FDA-cleared product allows surgeons to fuse a dataset to a patient on a surgical table so they can scroll through the slices to find the exact locations in the underlying tissues.
In February 2018, the National Institutes of Health (NIH) awarded a $2.2 million research grant to the company SentiAR Inc. to advance its work to design AR software to improve visualization in cardiac surgeries and interventional procedures. The grant was awarded following peer review by a panel of cardiologists, engineers and other experts. SentiAR will receive the funding in milestone installments as it advances its platform. It uses the Microsoft heads-up display, allowing physicians to view, measure and manipulate real-time holographic images of the patient’s heart during procedures while still being able to clearly see around the room. It uses real-time navigation data feeds rather than magnetic resonance imaging (MRI) or computed tomography (CT), and displays it as a hologram. SentiAR was hoping to have its technology submitted for FDA review in 2019.
Related Content:
VIDEO: Implementing CZT SPECT Cardiac Protocols to Reduce Radiation Dose
VIDEO: Demonstration of Shimadzu's Trinias Interventional X-ray System
Three Resolutions Worth Keeping for a More Data-driven Radiology Practice


Chris Meenan
The New Year gives us a fresh start and license to dream of what “could be” if we consistently apply a little discipline to the process. We make goals to achieve and promises to do better. We chart courses of change and hope to arrive in new destinations. Yet, in spite of all this planning and strategizing, we often get stymied when it comes to implementation. It’s human nature.
In healthcare, I see this same sort of phenomenon occur when it comes to the goal of becoming a more data-driven imaging department. There is an increased focus in radiology departments to drive operational efficiency with better analytics tools and the application of new technologies to improve interpretation and business processes. Radiology leaders have a strong interest in using data to guide decision-making processes, improve workflow and meet operational goals. However, resolve often dissipates in the transition from data gathering, to intelligence insight, to action.
In imaging today, we face numerous challenges: continued volume growth, more data per clinical decision than ever before, changing reimbursement metrics — and with staff and radiologist shortages, less resources. These pressures are compelling us to unlock the value of our data and leverage it to identify and drive operational efficiencies in imaging. Research suggests that 60-65 percent of the annual spend in radiology is in operations and $10-12 billion of that is waste (such as wrong test, wrong protocol, etc.).1
There is a real opportunity for healthcare providers to achieve operational cost savings that can then be reinvested into patient care. To do that, there are three New Year’s resolutions every radiology department should make.
Resolution #1: Why Being “Data Aware” Is No Longer Enough
While most facilities are using some type of data reporting for operational, financial and clinical decision support, the typical process is limited in scope and very much manually driven. As a result, reporting is very labor and time intensive, not standardized, costly and most importantly — delayed.
Delayed reporting doesn’t make radiology departments agile. In our personal lives, we are accustomed to having access to real-time data, whether it’s traffic information on a route, weather alerts or flight updates. We all understand that it is not helpful to have a report that tells you what the traffic was two weeks ago in order to navigate the best route today. We like tools such as Google Maps because it gives us real-time driving directions and suggests meaningful savings (e.g. reduced drive time to our destination or avoiding routes without tolls).
Limited access to real-time data, and trying to drive change with spreadsheet-based reports, are some of the biggest challenges for radiology departments today. This type of reporting provides only historic information that is days or weeks old. Static reporting may make departments “data aware,” but it is no longer sufficient in today’s healthcare environment of value-based care.
Resolution #2: Use Real-time Analytics to Identify, Prioritize Improvement Gains
In order to become data-driven, radiology leaders must make better, faster decisions. To do that, we need to move beyond simple reporting — tools that provide a limited view of what has already happened — to next-generation tools that provide a broader view that can help us predict what will likely happen. Radiology leaders need intelligent insights that can help them optimize their operations and identify and support the best possible decision-making. The value of real-time data is that it helps radiology leaders identify and prioritize opportunities for both short- and long-term improvement gains.
Improving workflow through technologies that automate manual processes can help healthcare organizations focus their valuable time where it’s most needed. The benefits of data-driven practice management range from quality improvement or reporting, to resource planning and expansion. This includes automating reports for appropriate MACRA reimbursement, tracking system utilization or staff efficiency, addressing scheduling backlogs and patient wait times, understanding volume shifts per modality across multiple locations, and more.
Specifically, meaningful operational analytics tools can help the radiology department improve workflow, reduce manual processes, and achieve gains in many areas such as:
• Repeat imaging or rescans — by examining issues to reduce the need for repeat imaging in order to reduce cost and increase patient safety and workflow productivity;
• Referral volume and patterns — by understanding which type of services are most needed for a hospital’s particular patient population to increase revenue;
• MACRA reimbursement — by reducing or removing manual effort to track appropriate quality measures with automated tracking; and
• Staff experience — by helping residents understand meaningful changes to preliminary reports to improve trainee experience and satisfaction.
The drive toward value-based care and improved efficiencies is forcing clinical leaders to seek the next generation of operational analytics tools. Advances in new technologies such as analytics, business intelligence, machine learning and artificial intelligence are making the tracking and automation of tasks possible. We see an increased application of next-generation analytics, machine learning and artificial intelligence to business processes and clinical outcomes improvement, in addition to the strong benefits to the diagnostic and interpretative process already underway.
However, there’s an internal change management component to the process as well that’s equally important.
Resolution #3: Data Analytics Can Change the Way You Run Business
We often don’t stick to our New Year’s resolutions because change is hard. We fall back to old habits or revert to what we’ve always done because it’s comforting, it’s easier, it’s worked. The first step to keeping a resolution is to believe that it can be done. In your mind, see the benefits of an aligned organization working together to meet goals and drive improvements. Envision a new departmental culture where everyone has a shared view of challenges and accountability to solve problems and sustain improvements.
A department that is empowered not just by data, but by meaningful insights can drive change. Operational analytics tools can offer many of these benefits today and will increasingly provide more capabilities over the next several years.
When clinical leaders can access the information they need in real-time, they can move past historic report shuffling and spreadsheet work to focus their attention on solving problems. They can find and share insights across their leadership teams to drive action and change.
There are real examples of organizations achieving meaningful improvements with data analytics. For instance, we worked with a large nonprofit health system whose vision was to enhance productivity, improve the patient and provider experience, and deliver value-based care. Working closely with the leadership team, we conducted an assessment across 16 of the provider’s radiology departments, benchmarking and analyzing patient volumes and equipment utilization, as well as referral and market growth.
We worked with them to establish key performance indicators (KPIs) and create workflow tools to track and drive improvements. With this analysis, we helped the provider identify the potential to significantly save on length-of-stay costs, improve magnetic resonance imaging (MRI) facilities efficiency by 10-20 percent to drive additional volume and revenue, and improve the overall patient and staff experience.2 This project and experience with our advanced analytics team and technology helped them achieve a clear view of the opportunities, a tangible measure of what they would gain and the evidence they needed to move forward toward action.
Using data to guide decision-making processes and workflows can be a big transition, but it is key to surviving in a value-based care world. Data provides deeper insights and objectivity in setting operational goals, rather than relying on “gut instinct” alone to make business decisions. With reimbursement increasingly tied to quality metrics, radiology departments must drive better operations not only for improved efficiencies but for financial health as well. When radiology practices are able to easily gather all the data from the entire imaging value chain, they are in a better position to understand issues, solve problems, predict behavior and achieve improvement gains on a continuous basis. That’s a New Year’s resolution worth keeping!
References
1. Peer60; Unnecessary Imaging. https://reactiondata.com/wp-content/uploads/2015/02/peer60-unnecessaryimaging.pdf
2. Results from case studies are not predictive of results in other cases. Results in other cases may vary.
Chris Meenan is the general manager of performance solutions, in the radiology solutions group at Philips. Formerly CEO of Analytical Informatics, Inc., before joining Philips in 2017, Meenan has a background in the design, implementation and support of innovative technologies in both commercial and clinical environments. He brings more than 15 years of technical leadership and business experience to the delivery of high-performance clinical information systems in healthcare. Meenan
has served on the faculty, as an assistant professor at the University of Maryland School of Medicine, as a board member for the Society for Imaging Informatics in Medicine (SIIM) and is currently a member of the Board of Trustees for the American Board of Imaging Informatics (ABII).
VIDEO: Managing a Multi-site Radiology Practice With AI-based Workflow
ACC.19 Future Hub Hosts “Shark Tank” of Emerging Technologies In Cardiology

Presenter delivers pitch at last year’s ACC Future Hub. This year during ACC.19, entrepreneurs will pitch software and hardware specific to cardiology in two categories– artificial intelligence and digitally enabled medical devices. (Image courtesy of ACC)

Greg Freiherr
Immersed in a Shark Tank-like atmosphere, entrepreneurs will pitch ideas for new technologies and services at ACC.19, this year’s annual meeting of the American College of Cardiology.
The first pitches will be delivered Sunday morning, March 17, beginning at 9:45 am for artificial intelligence (AI) applications in cardiology. A second wave will be tossed the next morning, also at 9:45 am, regarding digitally enabled medical devices. Panels of judges, along with an on-site audience and viewers on Facebook Live, will vote to determine the winner in each category.
The appeal of these challenges will be similar to what attracts viewers to the television show Shark Tank, said AI challenge moderator Andrew M. Freeman, M.D..
“You get people with very pointed and intense questions,” said Freeman, who moderated an “innovation challenge” at last year’s ACC meeting. The director of clinical cardiology at National Jewish Health, an academic hospital/clinic in Denver, compared his duties at this year’s AI challenge to those of a game show host. Primarily, he will try to engage the audience with presenters and keep pitches on schedule.
Freeman was chosen as a moderator, he said, partly for his experience moderating other ACC events, as well as for his passion about medical technology and interest in new ideas to manage cardiac patients. In his practice at National Jewish Health, Freeman integrates evidence-based medicine and plant-based diet, the latter of which has earned him the “Vegan Cardiologist” nickname.
Future Hub at ACC19 To Go Beyond Challenges
The challenges in these two categories — AI and digitally enabled devices — will highlight the ACC’s Future Hub, which aims “to inform, educate and inspire ACC.19 attendees by exposing them to the latest innovations in digital health, medical devices and big data,” according to its website. Presentations, including ones on the implications of technology for the doctor-patient relationship, will go on before and after the challenges.
Freeman described ACC’s Future Hub as a unique complement to the scientific sessions going on during the meeting: “I think if anyone is curious about the future of cardiology or medicine, it is certainly a way to get a glimpse of it.”
All Hub presenters will address how emerging technologies might transform cardiovascular practice. Kiosks located in the Future Hub Learning Destination (Expo Hall, #146) will provide individualized hands-on opportunities. The theater in the Hub, where the one-hour challenges will happen, will serve up TED-style talks; small panel discussions and debates on future-based issues; and exhibitor demonstrations.
In Search of Angel Investors
To host the challenges, ACC is partnering with AngelMD, self-described as “a healthcare investment community” that combines healthcare startups with physicians, investors, and industry “with the goal of creating successful outcomes.”
The winner of each challenge will be profiled on the AngelMD website. The exposure, Freeman said, could translate into “hundreds of thousands or even millions of dollars” in investments. Winners will also receive kiosk space at next year’s Future Hub; exhibit space at ACC20; and a commemorative plaque.
Last year Challenges featured eight finalists, each with fledgling but promising products or services. They were selected from more than 60 applications assessed by a committee of experts provided by AngelMD. A major criterion for selection was the likelihood of product success.
This year’s two challenges will focus either on AI-based software or digitally enabled hardware. Four finalists in each category will pitch their products or services. They were chosen by a review committee from “dozens of applications,” according to Freeman, who served on the committee.
Greg Freiherr is a contributing editor to Imaging Technology News (ITN). Over the past three decades, Freiherr has served as business and technology editor for publications in medical imaging, as well as consulted for vendors, professional organizations, academia, and financial institutions.
Editor's note: In preparation for the upcoming American College of Cardiology (ACC) Conference on March 17, contributing editor Greg Freiherr begins the show coverage with this an exclusive series of articles and podcasts. This is the first article in a series of three.
Related Content:
Future Hub Showcases Latest Innovations in CV Care; Home to ACC.18 Innovation Challenge
ACC.19 Innovation Challenge Participants Announced